Certified SARS-CoV-2 kits
We offer Covid-19 kits for hospitals and governmentsOne and only FDA, CE certified Covid Kit in Turkey
Cellex qSARS-CoV-2 IgG/IgM Rapid Test
Our Covid Kits are FDA certified which means that there are no adequate, approved, and available alternatives to the emergency use of our product.
INTENDED USE
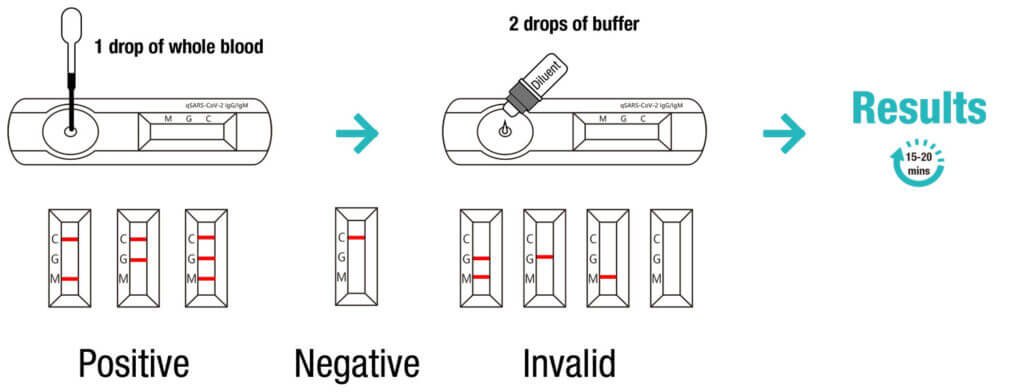
The Cellex qSARS-CoV-2 IgG/IgM Rapid Test is a lateral flow immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in serum, plasma (EDTA, citrate) or venipuncture whole blood specimens from patients suspected of COVID-19 infection by a healthcare provider.
The qSARS-CoV-2 IgG/IgM Rapid Test is an aid in the diagnosis of patients with suspected SARS-CoV-2 infection in conjunction with clinical presentation and the results of other laboratory tests.
Results from the qSARS-CoV-2 IgG/IgM Rapid Test should not be used as the sole basis for diagnosis.
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, to perform moderate and high complexity tests. Results are for the detection of SARS-CoC-2 antibodies. IgM antibodies to SARS-CoV-2 are generally detectable in bold several days after initial infection, although levels over the course of infection are not well characterized. IgG antibodies to SARS-CoV-2 become detectable later following infection. A CLIA categorization of this device would be consistent with other serology lateral flow moderate complexity devices.
Negative results do not preclude SARS-Cov-2 infection and should not be used as the sole basis for patient management decisions. IgM antibodies may not be detected in the first few days of infection; the sensitivity of the qSARS-Cov-2 IgG/IgM Rapid Test early after infection is unknown.
False positive results for IgM and IgG antibodies may occur due to cross-reactivity from pre-existing antibodies or other possible causes.
At this time, it is unknown for how long IgM or IgG antibodies may persist following infection.
For prescription use only. For in vitro diagnostic use only. For emergency use authorization use only.
Reach out to us via the form below and get the fastest Covid tests on the market.
Documents
Contact us
We are
Professional
Entrepreneurs
Professional
Entrepreneurs
Professional
Entrepreneurs
2019-2021 © Copyright – Montenegronline.com